These models provide an opportunity to study tumors in a whole organism, allowing researchers to observe how cancers develop, interact with the immune system, and respond to different treatments. The use of animal models is critical for preclinical testing of new therapies and for gaining insights into the genetic, molecular, and environmental factors that influence cancer.
Types of Animal Tumor Models
There are several types of animal tumor models, each with its own strengths and weaknesses. These models include syngeneic models, xenograft models, patient-derived xenografts (PDX), genetically engineered mouse models (GEMMs), and orthotopic models.
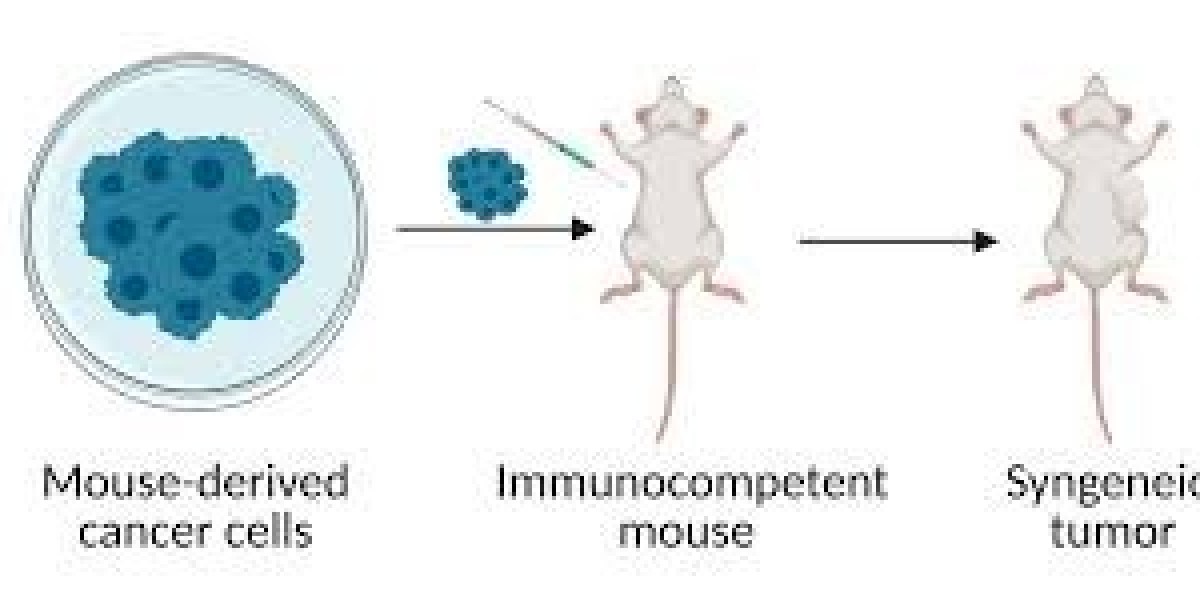
1. Syngeneic Tumor Models
In syngeneic tumor models, cancer cells from one animal are implanted into a genetically identical animal. This ensures that the immune system of the host is compatible with the tumor cells, enabling researchers to study the tumor’s interaction with the immune system.
- Applications: These models are particularly useful for studying the role of the immune system in cancer development, testing immunotherapies, and evaluating the efficacy of treatments that rely on immune responses.
- Advantages: They provide insights into immune-based therapies and tumor-immune system interactions.
- Limitations: These models are limited to certain species and tumor types.
2. Xenograft Models
In xenograft models, human cancer cells are transplanted into immunocompromised animals, usually mice, that lack a functional immune system. This allows the human cancer cells to grow and develop without being rejected by the host’s immune system.
- Applications: Xenograft models are widely used in preclinical cancer research, particularly for testing human-specific therapies, studying tumor progression, and understanding how human tumors behave in a living organism.
- Advantages: They offer a more human-like environment for studying tumor biology compared to in vitro models.
- Limitations: The absence of a functional immune system in these animals limits the study of immune-based therapies.
3. Patient-Derived Xenograft (PDX) Models
PDX models are created by implanting human tumor tissues directly into immunocompromised mice. These models preserve the genetic and molecular features of the original patient’s tumor, making them highly relevant for testing personalized treatments.
- Applications: PDX models are useful for studying tumor heterogeneity, metastasis, and drug resistance, and they are particularly valuable in developing personalized medicine strategies.
- Advantages: They offer a closer approximation to human cancer biology and retain the heterogeneity of the original patient tumor.
- Limitations: PDX models are resource-intensive, require patient-derived tumor samples, and are time-consuming to establish.
4. Genetically Engineered Mouse Models (GEMMs)
GEMMs are genetically modified mice that develop cancer due to specific mutations that mimic the genetic alterations found in human cancers. These models allow researchers to study cancer development from its initiation to its progression.
- Applications: GEMMs are essential for understanding the genetic and molecular mechanisms behind cancer. They are used to explore the role of specific genes in tumorigenesis, metastasis, and drug resistance.
- Advantages: GEMMs provide insights into the early stages of cancer development and the impact of genetic alterations.
- Limitations: GEMMs can take a long time to develop, and they may not fully replicate the complexity of human cancers.
5. Orthotopic Tumor Models
In orthotopic models, cancer cells are implanted directly into the organ from which they originated. This allows for more realistic tumor growth and metastasis, as the tumors develop in their natural environment.
- Applications: Orthotopic models are useful for studying cancer metastasis and the tumor microenvironment. They are also employed in testing therapies that target specific organs or tissues.
- Advantages: These models provide more physiologically relevant information about tumor growth and metastasis.
- Limitations: They are technically challenging to set up and often require surgical implantation of tumor cells.
Applications of Animal Tumor Models
Drug Discovery and Testing: Animal tumor models are essential for preclinical testing of cancer therapies, including chemotherapy, targeted therapies, and immunotherapies. They allow researchers to evaluate the effectiveness and safety of new drugs before they are tested in human clinical trials.
Immunotherapy Research: With the growing importance of immunotherapies like immune checkpoint inhibitors, CAR-T cell therapy, and cancer vaccines, animal models are invaluable for evaluating these treatments. They allow scientists to explore how tumors interact with the immune system and assess the potential of immune-based therapies.
Metastasis Studies: Understanding cancer metastasis is critical for developing treatments to prevent the spread of cancer to other organs. Animal models, particularly orthotopic and GEMMs, are used to study the complex processes of metastasis and the factors that drive cancer spread.
Tumor Microenvironment Research: The tumor microenvironment (TME) plays a crucial role in cancer progression and therapy resistance. Animal models help researchers study the interactions between tumor cells, immune cells, blood vessels, and surrounding tissues to identify new therapeutic targets.
Personalized Medicine: By using patient-derived xenografts (PDX) and organoid models, researchers can test various treatments on tumors derived from individual patients. This approach allows for the development of personalized treatment strategies that are tailored to the unique characteristics of each patient’s cancer.
Challenges and Limitations
Species Differences: One of the primary limitations of animal tumor models is that they do not always perfectly replicate human cancer biology. Tumors in animals can behave differently than in humans, and drug responses may not always translate effectively from animal models to human patients.
Immune System Differences: Many animal tumor models use immunocompromised animals, which do not have a fully functional immune system. While this allows for the study of tumor growth, it limits the ability to study immunotherapies, which rely on an active immune system.
Ethical Considerations: The use of animals in research raises ethical concerns regarding animal welfare. Researchers are continuously working to reduce the number of animals used in experiments and to explore alternative models, such as 3D cultures, organoids, and computational models.
Cost and Time: Animal tumor models can be expensive and time-consuming to establish and maintain. For example, PDX models require patient tumor samples and take months to develop. The cost of maintaining these models may limit their use in large-scale studies.
Future Directions
As cancer research progresses, the development of more sophisticated and human-relevant tumor models is on the horizon. Some key areas of advancement include:
- Humanized Animal Models: By introducing human immune cells or humanized tissue into animals, researchers are working to create models that better mimic human immune responses and cancer progression.
- Organoid and 3D Cultures: Advances in organoid and 3D culture technology are providing more accurate representations of human tumors and offering a platform for high-throughput drug screening.
- CRISPR and Gene Editing: The use of CRISPR technology is allowing for the precise editing of genes in animal models, enabling researchers to study the effects of specific genetic mutations and to create more accurate models of human cancer.
Conclusion
Animal tumor models remain a cornerstone of cancer research, providing essential insights into the biology of cancer, the tumor microenvironment, and the effectiveness of new therapies. Despite their limitations, these models continue to play a critical role in advancing cancer treatment and improving our understanding of the disease. With ongoing advancements in technology and the development of more human-like models, the future of cancer research holds great promise for more effective treatments and better patient outcomes.