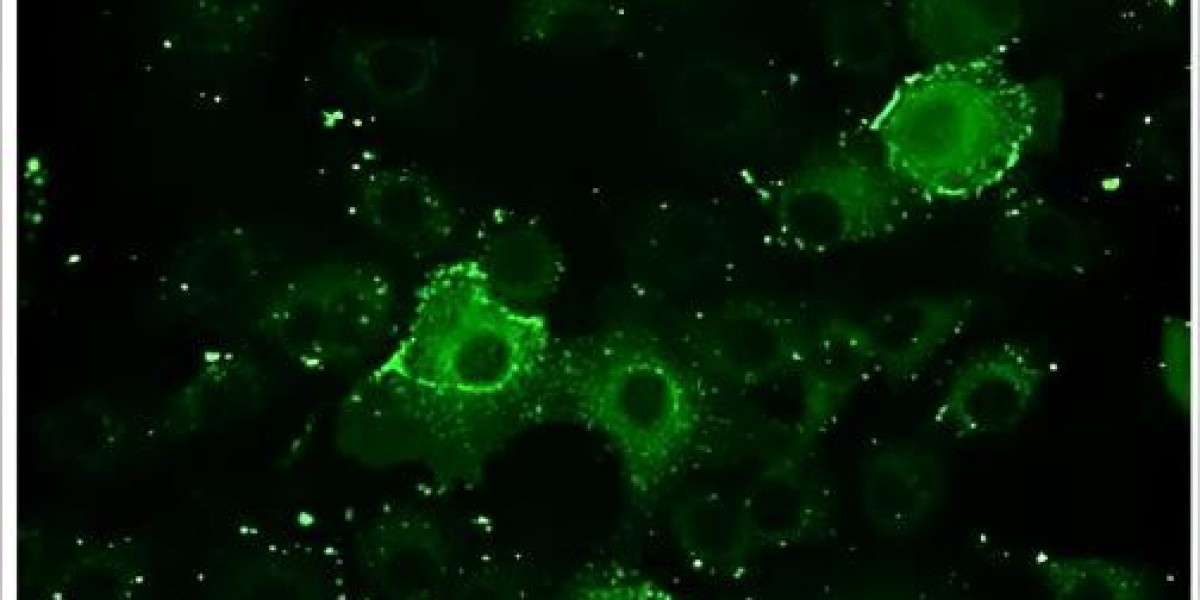
As an expert in providing solutions to assist virology and microbiology research, Creative Diagnostics is pleased to announce the launch of its state-of-the-art Immunofluorescence Assay (IFA) Testing services to support virology research. The new services provide researchers with a critical tool to confirm the presence or absence of antibodies against specific viruses, commonly employed in an ELISA or MFIA test.
The IFA is a standard technique in virology and is commonly used to confirm the presence of infectious antibodies to specific viral antigens. The technique is typically used after an enzyme-linked immunosorbent assay (ELISA) or multiple fluorescence immunoassay (MFIA) to confirm a positive result. Creative Diagnostics is committed to providing researchers with the most advanced technology and expertise to advance antiviral research. These new IFA testing services offer a cost-effective and reliable solution and unparalleled support in IFA development and application, helping researchers accelerate their antiviral programs.
Specifically, in the first incubation step, specific antibodies in the diluted sample bind to the solid-bound antigen. In the next step, a fluorescein-labeled antibody (conjugate) binds to the specific antibody in the sample. The complex is clearly visible under a fluorescence microscope when excited at the appropriate wavelength. Test processing can be manual, semi-automated, or fully automated.
Creative Diagnostics’ IFA testing services offer several advantages. They are relatively inexpensive to perform, and the morphology and location of the fluorescence can be evaluated to distinguish specific from non-specific reactions. Additionally, these assays are validated with a panel of titered sera to ensure sensitivity, and they allow the detection of IgG or IgM antibodies. Finally, the presence of positive and negative control cells provides a built-in check for the accuracy of the test. These IFA testing services offer a powerful combination of experience, flexibility, and accuracy to accelerate critical research efforts.
In addition, Creative Diagnostics has a team of experienced scientists who are experts in IFA development and understand the importance of accurate and reliable IFA tests. By combining its IFA testing expertise with its in-depth knowledge of antiviral research, Creative Diagnostics is dedicated to working closely with clients to develop assays that meet their specific needs.
Utilizing cutting-edge technology, Creative Diagnostics offers a wide range of antiviral services to evaluate the inhibitory activity of inhibitor candidates. Moreover, a variety of customized antiviral assays are available to elucidate the action mechanism of novel antiviral drugs. Creative Diagnostics has developed and optimized novel antiviral assays for multiple viruses. All antiviral assays can be tested for cytotoxicity in transformed or primary cells to ensure accurate and reliable results.
Creative Diagnostics offers researchers IFA Testing services to accelerate breakthroughs in the field of virology. For more information about the IFA Testing, please visit https://antiviral.creative-diagnostics.com/ifa-testing.html.
About Creative Diagnostics
Headquartered in New York, Creative Diagnostics is a consulting and experimental service provider specializing in virology and microbiology. The company provides comprehensive solutions to conquer obstacles in virology and microbiology research, from high-security infrastructure provision, biosafety regulation elucidation, to expert viral system assistance.